Over the past few weeks, Congress has made significant progress toward finalizing the Build Back Better Act, a major budget reconciliation bill that represents the only likely vehicle for major policy changes to the Medicare program this year. Although significant portions of the bill are still in flux, there has been recent consensus around certain key provisions affecting the Medicare and Part D programs. Please refer to our detailed article for more information.
Drug price negotiation
The draft bill includes a provision for Medicare drug price negotiation. Some specifics include:
- Both Medicare Part B and D drugs would be eligible, with drugs targeted based on total expenditures.
- Eligible drugs include those from the top 50 highest-spend single source Part B drugs, the 50 highest-spend single source Part D drugs, and single-source insulins.
- A list of up to 10 drugs would be negotiable in 2025, increasing by 15 drugs in both 2026 and 2027, and 20 drugs in 2028. Our understanding is the number of negotiable drugs would be cumulative.1 Thus, a total of 60 drugs would be subject to price negotiation by 2028.
- Prices for all insulin products would be negotiable, in addition to the select number of drugs noted above.
- Small molecule drugs would be eligible for price negotiation nine years after launch, while biologics would be eligible after 13 years.
- The legislation provides guardrails for the price negotiation, indicating the minimum discounts would be:
- 25% for a short-monopoly drug (< 12 years since launch)
- 35% for post-exclusivity drug (> 12 years and < 16 years since launch)
- 60% for a long-monopoly drug (> 16 years since launch)
- Small biotech drugs would be exempt from price negotiation through 2027. This includes drugs that account for less than 1% of expenditures in Part B and D and comprise more than 80% of expenditures across all of the manufacturer’s drugs.
- Manufacturers would pay a penalty equal to 10 times the amount charged above the negotiated price (10 x Units Dispensed * [Actual Price – Negotiated Price]) for not providing eligible entities the maximum fair price during a period of agreement.
- Manufacturers that decline negotiation for a selected drug will pay a variable tax of two times to 19 times the daily sales.
Part D benefit redesign
The draft bill also proposes a redesign of the Part D benefit. Some specifics include:
- The majority of the redesign would start to be effective January 1, 2024 (not for the upcoming 2023 bid cycle).
- The redesign includes a maximum-out-of-pocket (MOOP) of $2,000 and elimination of the coverage gap. Currently, the true-out-of-pocket (TrOOP) cost is $7,050 for 2022.
- Federal reinsurance would decrease from 80% for all drugs to 20% for applicable (typically brand) drugs and 40% for nonapplicable (typically generic) drugs.
- The BBB introduces a new pharmaceutical manufacturer discount program that reflects 10% of applicable drug costs above the deductible and below the MOOP, and 20% of drug costs above the MOOP.
- The pharmaceutical manufacturer discount would be phased in for:
- Low-income subsidy (LIS) beneficiaries for drugs from a “specified” manufacturer, defined as having expenditures from all of their drugs reflect less than 1% each of all Part B and D expenditures.
- All beneficiaries taking drugs from a “specified small” manufacturer, defined as having (a) expenditures from all their drugs reflect less than 1% each of all Part B and D expenditures, and (b) one drug that accounts for more than 80% of all their associated expenditures.
- For drugs subject to phase-in, the pharmaceutical manufacturer discount phase-in schedule is as follows (also summarized in Figure 2 below), applied as a percentage of allowed cost in the respective benefit phase:
- Above deductible and below MOOP: 1% (2024), 2% (2025), 5% (2026), 8% (2027), and 10% (2028)
- Above MOOP: 1% (2024), 2% (2025), 5% (2026), 8% (2027), 10% (2028), 15% (2029), and 20% (2030)
- New pharmaceutical manufacturer discount program would not apply to drugs selected for price negotiation.
- Defined standard member cost sharing would decrease from 25% to 23% between the deductible and MOOP.
- Part D national average member premium would be revised from 25.5% to 23.5% of total program cost.
- Out-of-pocket costs for insulin products would be capped at $35 per month for all plans starting in 2023.
- Beginning in 2025, beneficiaries would be able to opt-in to “smooth” cost sharing throughout the year instead of following the standard benefit design. Member would pay fixed cost-sharing amounts each month.
Drug inflation rebates
The draft bill includes a provision for manufacturers to pay inflation rebates on single source brand and biologic drugs in both the Medicare and commercial markets if prices increase at a faster rate than inflation. A few key notes:
- Drug inflation would be benchmarked relative to prices on October 1, 2021, trended forward by the Consumer Price Index for All Urban Consumers (CPI-U).
- Any price increases in excess of CPI-U changes over this time period would be paid as an inflation rebate.
- Inflation rebates would apply to both the commercial and Medicare markets.
- Part B member coinsurance would be based on the inflation-adjusted price.
- Inflation rebates would be paid directly to the Part B and D trust fund.
- Inflation rebates could go into effect by 2023 (January for Part D, July for Part B).
Other changes
- The draft bill would indefinitely delay the point-of-sale rebate rule.2
- Medicare fee-for-service (FFS) coverage would expand to include hearing but would continue to exclude most dental and vision services.
- Starting in 2024, beneficiaries would pay no cost sharing for vaccines covered under Part D.
For more information or to understand the impact of these items, contact your Milliman consultant.
Exhibits
Figure 1: Part D comparison of cost sharing by stakeholder: Current vs. revised Part D design
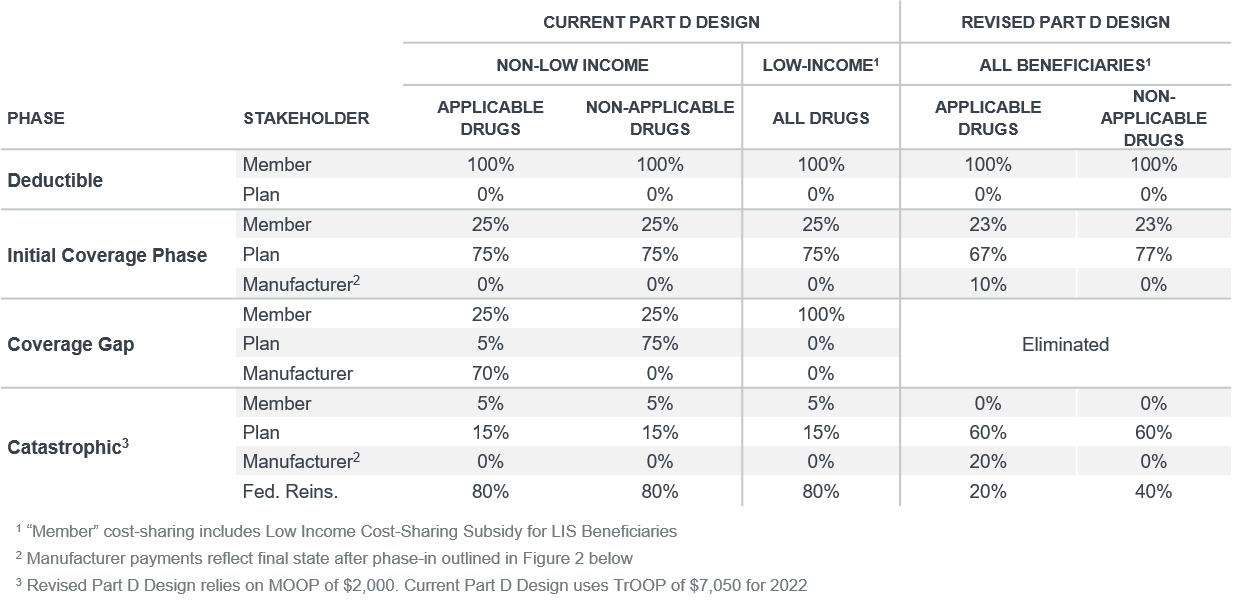
Figure 2: Manufacturer discount phase-in for LIS beneficiaries for “specified” and all beneficiaries for “specified small” manufacturers
1 Owens, C. & Herman, B. (November 5, 2021). Medicare drug negotiations may not be as limited as we thought. Axios. Retrieved November 24, 2021, from https://www.axios.com/democrats-medicare-prescription-drug-negotiations-bf94bd22-3aad-42c1-a9f1-12c9183814c0.html.
2 The full text of the draft bill is available at https://www.federalregister.gov/documents/2020/11/30/2020-25841/fraud-and-abuse-removal-of-safe-harbor-protection-for-rebates-involving-prescription-pharmaceuticals.
