Background
The escalating costs of the U.S. healthcare system have been a subject of concern for decades, with drug spending driving nearly half of the surge in healthcare costs recently.1 Drug spending is made up of unit cost (the cost per dose) and utilization (the number of doses dispensed). The complex nature of pharmaceutical pricing and the current patent system contributes to high unit costs until therapies have generic competition. Prescription drug utilization patterns, particularly for chronic diseases, have also led to increased drug spending. The implications of these rising costs are widespread. Patients face increased financial burdens, often having to choose between essential medications and other basic needs. Healthcare providers struggle to balance the delivery of quality care with cost containment strategies, while patients, employers, and taxpayers ultimately bear the brunt of these escalating costs.
To address the issue of soaring drug spending, a multifaceted approach will be necessary. This white paper focuses on one approach for improving drug utilization management: precision medicine through a method known as uplift modeling. Uplift modeling is a novel approach—already popular in other industries such as marketing and advertising2 and education3—that could be a great candidate to finally deliver on the promises of precision medicine.
Precision medicine, a personalized approach
Precision medicine, also known as personalized medicine, is a rapidly evolving field that tailors medical treatment to the individual characteristics, needs, and preferences of each patient. This approach utilizes a combination of different types of data to create a more precise diagnosis and treatment plan.
With the dramatic expansion of big data and advanced analytics, precision medicine is potentially poised to revolutionize healthcare delivery, leading to improved patient outcomes and potentially significant cost savings.
However, the transition to this new model of care also presents a host of challenges, including data privacy concerns, regulatory hurdles, and ethical considerations that are further discussed later.
Precision medicine complements and enhances evidence-based medicine to reduce some of the health and financial risks common in U.S. healthcare. Evidence-based medicine is the conscientious, explicit, and judicious use of current best evidence in making decisions about the care of individual patients. It involves integrating individual clinical expertise with the best available external clinical evidence from research. However, that research is created by—and for—an ecosystem that often has incentives misaligned with determining which treatments will be the most successful for each individual patient. For example, from the perspective of prescription medications or medical devices approved by the U.S. Food and Drug Administration (FDA), companies are financially incentivized to get their treatment approved for the largest population possible, even if a small subset of that population is driving most of the benefit. By combining precision medicine and evidence-based medicine, healthcare providers can make treatment decisions that are both grounded in the latest scientific research and tailored to the individual patient's needs and circumstances. This could potentially lead to improved patient outcomes, greater patient satisfaction, and more cost-effective healthcare delivery.
Pharmacogenomic testing case study
Pharmacogenomic (PGx) testing is a key tool in precision medicine that can help determine how an individual's genetic makeup may influence their response to certain drugs. For instance, consider the case of a patient being treated for depression with a common selective serotonin reuptake inhibitor (SSRI) like fluoxetine. The typical route is to try an SSRI for four to six weeks, then evaluate the impact on symptoms. Despite taking the medication as prescribed, a patient continues to experience depressive symptoms. This could lead to an adjustment in dose, switching to another SSRI, or trying a different type of antidepressant for another four to six weeks. Sometimes, it can take several adjustments and months of delay before the patient experiences a relief of symptoms.
To speed up this timeline, PGx testing can be used to understand the patient's unique genetic profile and how it might impact the metabolism and effectiveness of the drug. The test may reveal that the patient has a variant of the CYP2D6 gene, an enzyme responsible for metabolizing many drugs—including SSRIs. This genetic variation can cause the patient to metabolize fluoxetine more quickly than average, reducing the drug's effectiveness. Based on this information, the healthcare provider might decide to prescribe a higher dosage of the medication or switch to a different drug that is metabolized by a different enzyme. This example underscores that not every drug is equally helpful for every patient, even though this patient would be eligible for fluoxetine according to the FDA-approved indication. Precision medicine through PGx is one common strategy for optimizing treatment outcomes.
Enhancing precision medicine with uplift modeling
Uplift modeling is a machine learning technique used to estimate the impact of an action or treatment at the individual level and could be used to help quantify the differences in medication effectiveness when PGx testing is available. Additionally, it could potentially be used to support precision medicine by identifying individuals most likely to benefit based on variables that are easier to measure and track (e.g., age, gender, etc.). For example, imagine an online pharmacy owner who just designed a new advertisement with a 15% discount for an over-the-counter product. The owner will want to know if the advertisement led to more sales and how profitable the campaign was overall. Uplift modeling could help the pharmacy owner identify which customers are likely to purchase the product regardless of the advertisement and which customers are likely to make a purchase because they received the discount. With this information, the pharmacy owner could optimize their limited resources by ensuring that the discounts were prioritized for those “persuadable” customers rather than spread across all customers, including those who would have made the purchase anyway.
This technique could be used in a similar fashion to identify the patients that are most likely to benefit from a healthcare intervention such as a prescription drug or medical device, or even estimate the differences in effectiveness between multiple candidate treatments (e.g., dosages). This is a large departure from traditional methods of estimating effectiveness that rely on estimating the average treatment effect for a group of people and comparing it to that of a control group. Further, uplift modeling can be used to identify subpopulations of people with similar characteristics that responded to the treatment in similar ways. This process can offer data-driven insights as to why some groups might not have responded well—or even why some groups might have had an adverse reaction.
Uplift modeling on high-cost drugs
Ozempic, Mounjaro, Wegovy, and other glucagon-like peptide-1 (GLP-1) agonists have made major headlines in recent years for their ability to treat type 2 diabetes and, in some cases, promote weight loss.4 However, emerging data shows that not everyone can expect to see the same effects: "There is wide variation in weight loss on these types of drugs, called GLP-1s. Doctors say roughly 10% to 15% of people who try them are 'non-responders'... about 14% of patients lost less than 5% of their body weight. About a third lost less than 10%."5 This raises the question—is there some way that we could reasonably predict which individuals might significantly benefit from GLP-1s? Or taken further, for individuals who might only benefit slightly, is there some data-driven framework that could help insurers find a reasonable compromise between full coverage and no coverage? In this case, the simplest definition of “uplift” would refer to the estimated probability that an individual experiences any amount of weight loss as a result of taking a GLP-1, although more advanced methods might estimate the number of pounds lost instead of a binary “lost-weight-or-didn’t” outcome. A simple way to do this would be to train two separate statistical models to predict whether someone was going to lose weight—one on the treatment group and one on a control group. Afterward, each individual is passed through both models to predict the probability of their weight loss if they had received the treatment and if they hadn’t. Then the difference in the two probabilities would be the estimated effect of the treatment. Figure 1 is an example of a potential output from uplifting modeling.
Figure 1: Cumulative gains chart for two GLP-1s (simulated data)
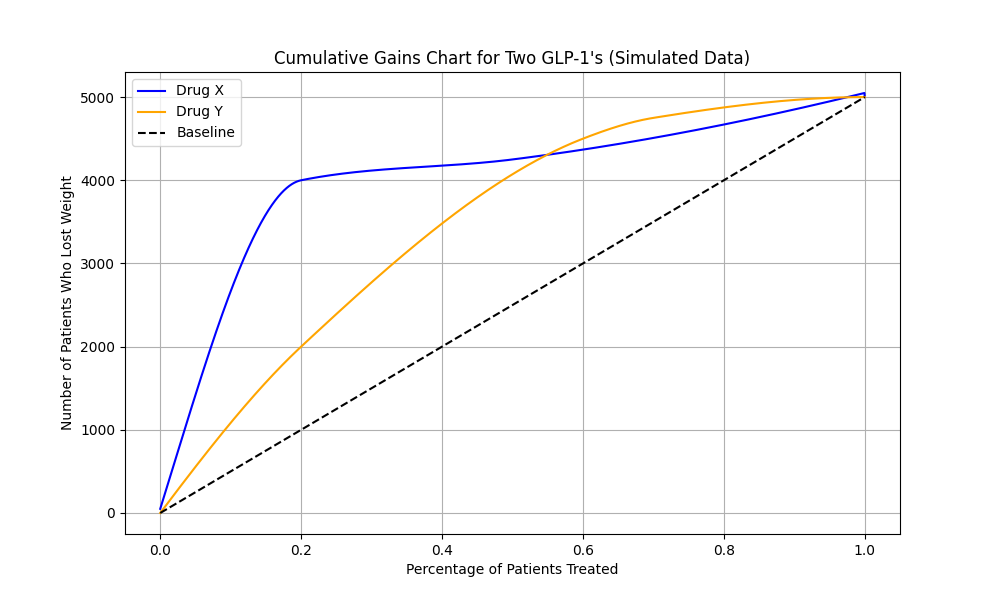
Cumulative gains charts like the one above are used to evaluate the estimated heterogeneity (i.e., the extent to which a treatment affects different people in different ways) of a treatment plan by comparing the percentage of a population that received the treatment (the x-axis) with the number of people who successfully lost weight (the y-axis). In this simulated example, we assume that approximately 5,000 people across the study lost weight. After training the uplift model, we predict the probability of losing weight for each person that received Drug X or Drug Y and then order them from highest probability to least probability across the x-axis. The dashed line establishes a baseline effectiveness where we assigned treatments randomly to patients instead of targeting those with the highest probability of losing weight. The blue line representing Drug X shows that targeting the specific individuals who are predicted to benefit most [from Drug X] is very effective for about 20% of the population, but less effective for the other 80%. Conversely, the yellow line representing Drug Y shows broader effectiveness when targeting a larger portion of the population (specifically between 50%-90%). However, in both cases, we see a large benefit over the baseline expectation, highlighting the usefulness of an uplift-based targeting strategy.
What to do with the uplift modeling output
Utilizing this tool in an ethical and sensitive way is vital. As precision medicine becomes more prevalent, there is a potential for techniques such as these to exacerbate existing disparities in healthcare because of the data available for training these models, and the potential for using them to deny needed care. This is particularly relevant when considering cost and access to healthcare and information technologies, which could inadvertently widen the gap between different patient groups.6 Therefore, extra precaution should be taken to anticipate and address these challenges, ensuring that the benefits of precision medicine are accessible and equitable.
With this ethical context in mind, who could benefit from uplift modeling? In addition to patients who may benefit from getting placed on more effective treatment options sooner, the answer is everyone who is taking financial risk for healthcare spending, including:
How could these stakeholders use the results from an uplift modeling analysis? One high-potential application could be the placement of specific drugs on formularies and preferred drug lists. For example, drugs that are only likely to benefit about 10% of the eligible population might be placed on a higher formulary tier. Another option is using this information to guide targeted utilization management (UM). For a drug that benefits 10% of the eligible population, there may be minimal UM for those who benefit, while the other 90% may see increasing levels of UM or even coverage exclusions.
From a provider or healthcare technology perspective, this information could inform adjustments to prescribing guidelines or standard workflow options. Additionally, when treatment effects are highly heterogeneous across a population, uplift modeling can be used to estimate the most important drivers of the heterogeneity, helping patients make more informed treatment decisions. For example, if someone was considering a GLP-1 for weight management, but the model indicated that weight loss may be limited for their specific demographic or lifestyle characteristics, they might not choose to take the drug. In all cases, uplift modeling can promote data-driven decision-making and strategic nudges for clinical practice or as part of workflows in software products.
Ultimately, the goal is to reduce the barriers to valuable treatments and protect patients from interventions that have a low likelihood of benefit (that come with financial cost and possible side effects), not to use uplift modeling to deny care—a crucial reminder of the importance of a grounded and well-respected code of ethics.
Conclusion
The rising costs of healthcare in the United States are driven in part by high prices and inefficient healthcare utilization, becoming a significant challenge to patients, providers, and payers alike. Precision medicine promotes a more efficient utilization of existing healthcare resources—tackling rising costs and reducing the risk of overprescribing and overtreating.
While precision medicine offers the potential of improved patient outcomes and cost savings, its implementation is not without challenges, including data privacy, regulatory hurdles, and potential exacerbation of existing healthcare disparities. Therefore, a careful, ethical approach is necessary to ensure that the benefits of precision medicine are accessible and equitable. By integrating precision medicine with evidence-based practice, healthcare providers can make treatment decisions that are both clinically sound and personalized to each patient's unique needs. If adopted widely, this approach could revolutionize healthcare delivery and significantly mitigate the financial burden on the U.S. healthcare system. While a multifaceted approach will be necessary to truly address this complex issue, precision medicine, particularly through an innovative approach like uplift modeling, is a great place to start and has significant potential to address escalating healthcare costs.
Caveats and limitations
This report is intended for informational purposes only. Milliman makes no representation or warranties regarding the contents of this report. Likewise, readers of this report are instructed that they are to place no reliance upon this report that would result in the creation of any duty or liability under any theory of law by Milliman or its employees to third parties.
Throughout this paper, Milliman relied on data and other information provided by publicly available data sources. The estimates included in this paper are not predictions of the future; they are estimates based on the assumptions and data analyzed at a point in time. If the underlying data or other listings are inaccurate or incomplete, the results may also be inaccurate or incomplete. Milliman has not audited or verified this data and other information but has reviewed it for reasonableness.
Effectiveness analyses are highly dependent on the quality and completeness of the underlying data. It is always important to employ experimental design best practices when performing effectiveness studies. Even advanced statistical techniques, like uplift modeling, can perpetuate biases present in the training data. For example, if there is underlying selection bias in the creation of the treatment and/or control group(s) that is unmeasured, then any resulting analysis will also be biased.
The views expressed in this research paper are made by the authors and do not represent the opinions of Milliman, Inc. Other Milliman consultants may hold alternative views and reach different conclusions from those shown.
1 Bell, D., Gaal, M., Man, A., Clarkson, J., Liner, D.M., Naugle, A.L. (May 21, 2024). 2024 Milliman Medical Index, retrieved August 14, 2024, from https://www.milliman.com/en/insight/2024-milliman-medical-index.
2 Bamidele, T. & Mgbaja, U. (March 4, 2024). Enhancing Targeted Marketing Strategies: Interpretable Uplift Modeling to Identify Key Client Segments. PREPRINT (Version 1) available at Research Square, retrieved July 31, 2024, from https://doi.org/10.21203/rs.3.rs-4006839/v1.
3 Olaya, D., Vásquez, J., Maldonado, S., Miranda, J., & Verbeke, W. (July 2020), Uplift Modeling for Preventing Student Dropout in Higher Education, Decision Support Systems, Volume 134, 113320, ISSN 0167-9236. Retrieved July 31, 2024, from https://doi.org/10.1016/j.dss.2020.113320.
4 Gallagher, A. (May 23, 2024). Counsel Patients on Nutrition and Following a Balanced Diet Complementary to GLP-1s. Pharmacy Times. Retrieved July 31, 2024, from https://www.pharmacytimes.com/view/counsel-patients-on-nutrition-and-following-a-balanced-diet-complementary-to-glp-1s.
5 Reddy, S. (April 1, 2024). They Thought Ozempic Would Help Them Lose Weight. It Didn’t Work. Wall Street Journal. Retrieved July 31, 2024, from https://www.wsj.com/health/pharma/ozempic-weight-loss-drug-ineffective-why-6d7059bb.
6 Brothers, K.B. & Rothstein, M.A. (November 1, 2015). Ethical, Legal and Social Implications of Incorporating Personalized Medicine Into Healthcare. Per Med. Retrieved July 31, 2024, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4296905/.
